Πολώνιο
84
Po
Ομάδα
16
Περίοδος
6
Τομέας
p
Πρωτονίων
ηλεκτρονικά
Νετρονίων
84
84
126
Γενικές Ιδιότητες
Ατομικός Αριθμός
84
Ατομικό βάρος
[210]
αριθμός μάζας
210
Κατηγορία
Μεταλλοειδή
Χρώμα
ασημί
ραδιενεργο
Ναι
Named after Poland, native country of Madam Curie
Κρυσταλλικό σύστημα
απλος κυβος
Ιστορία
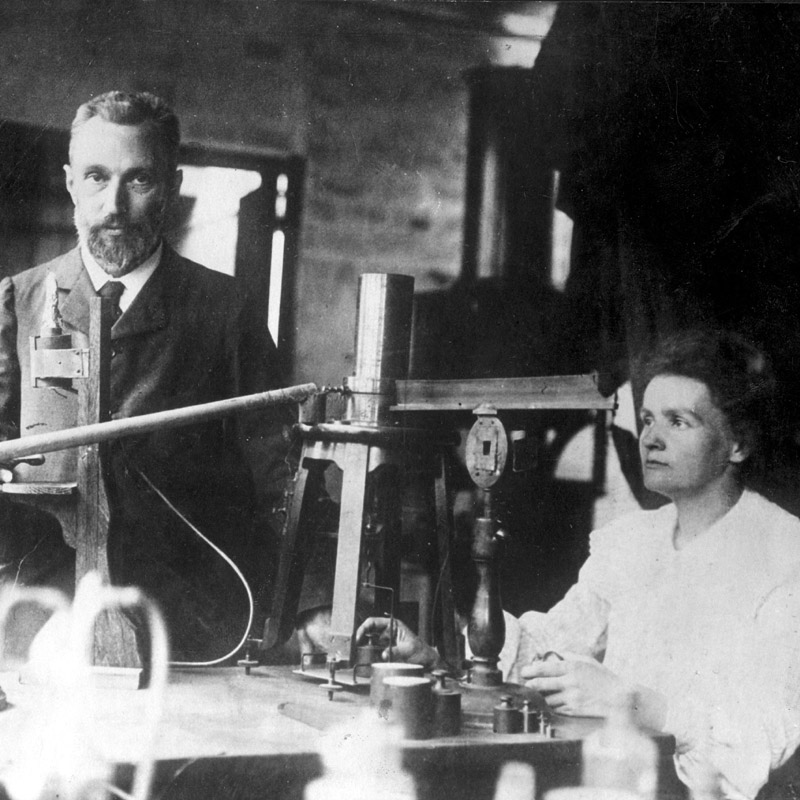
Polonium was discovered by Marie and Pierre Curie in 1898 in Paris.
This element was the first one discovered by the Curies while they were investigating the cause of pitchblende radioactivity.
The dangers of working with radioactive elements were not known when the Curies made their discoveries.
This element was the first one discovered by the Curies while they were investigating the cause of pitchblende radioactivity.
The dangers of working with radioactive elements were not known when the Curies made their discoveries.
Ηλεκτρόνια ανά κέλυφος
2, 8, 18, 32, 18, 6
Ηλεκτρονική διαμόρφωση
[Xe] 4f14 5d10 6s2 6p4
Polonium is obtained by irradiating bismuth with high-energy neutrons or protons
φυσικές ιδιότητες
φαση
Στερεά
Πυκνότητα
9,196 g/cm3
Σημείο τήξης
527,15 K | 254 °C | 489,2 °F
Σημείο βρασμού
1235,15 K | 962 °C | 1763,6 °F
θερμότητα σύντηξης
13 kJ/mol
Θερμότητα εξάτμισης
100 kJ/mol
Ειδική θερμοχωρητικότητα
- J/g·K
Αφθονία στον φλοιό της Γης
χωρις στοιχεια
Αφθονια στο συμπαν
χωρις στοιχεια
μοναδες CAS
7440-08-6
PubChem CID Number
χωρις στοιχεια
ατομικές ιδιότητες
Ατομική ακτίνα
168 pm
Ομοιοπολική ακτίνα
140 pm
Ηλεκτραρνητικότητα
2,00 (Κλίμακα Pauling)
Δυνατότητα ιοντισμού
8,417 eV
Ατομικός Αριθμός
22,23 cm3/mol
Θερμική αγωγιμότητα
0,2 W/cm·K
Καταστάσεις οξείδωσης
-2, 2, 4, 6
εφαρμογές
Polonium is used to eliminate static electricity produced during processes such as rolling paper, wire and sheet metal.
Polonium can be mixed or alloyed with beryllium to provide a source of neutrons.
It is also used in anti-static brushes to eliminate dust on photographic film.
Polonium can be mixed or alloyed with beryllium to provide a source of neutrons.
It is also used in anti-static brushes to eliminate dust on photographic film.
Το πολώνιο είναι εξαιρετικά επικίνδυνο και ραδιενεργό
Ισότοπα
Σταθερά Ισότοπα
-ασταθη Ισότοπα
188Po, 189Po, 190Po, 191Po, 192Po, 193Po, 194Po, 195Po, 196Po, 197Po, 198Po, 199Po, 200Po, 201Po, 202Po, 203Po, 204Po, 205Po, 206Po, 207Po, 208Po, 209Po, 210Po, 211Po, 212Po, 213Po, 214Po, 215Po, 216Po, 217Po, 218Po, 219Po, 220Po