Ράδιο
88
Ra
Ομάδα
2
Περίοδος
7
Τομέας
s
Πρωτονίων
ηλεκτρονικά
Νετρονίων
88
88
138
Γενικές Ιδιότητες
Ατομικός Αριθμός
88
Ατομικό βάρος
[226]
αριθμός μάζας
226
Κατηγορία
Αλκαλικές γαίες
Χρώμα
ασημί
ραδιενεργο
Ναι
From the Latin word radius meaning ray
Κρυσταλλικό σύστημα
Κυβικό στο κέντρο του σώματος
Ιστορία
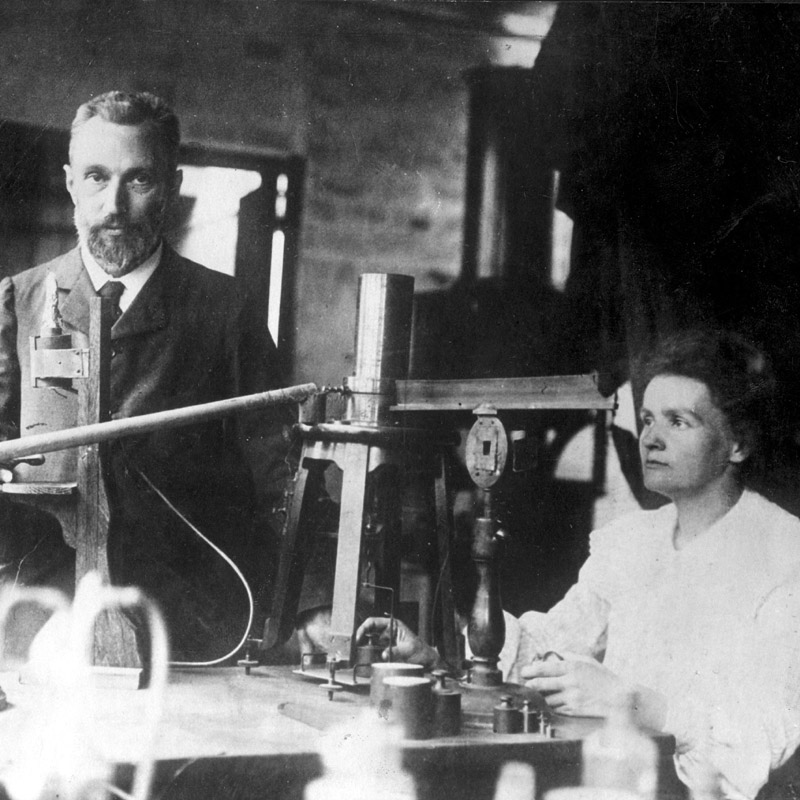
Radium was discovered by Marie Curie and Pierre Curie in 1898.
They extracted the radium compound from a uraninite sample.
Radium was isolated in its metallic state by Marie Curie and André-Louis Debierne in 1910 through the electrolysis of radium chloride by using a mercury cathode and distilling in an atmosphere of hydrogen gas.
They extracted the radium compound from a uraninite sample.
Radium was isolated in its metallic state by Marie Curie and André-Louis Debierne in 1910 through the electrolysis of radium chloride by using a mercury cathode and distilling in an atmosphere of hydrogen gas.
Ηλεκτρόνια ανά κέλυφος
2, 8, 18, 32, 18, 8, 2
Ηλεκτρονική διαμόρφωση
[Rn] 7s2
Radium imparts a carmine red color to a flame
φυσικές ιδιότητες
φαση
Στερεά
Πυκνότητα
5,5 g/cm3
Σημείο τήξης
973,15 K | 700 °C | 1292 °F
Σημείο βρασμού
2010,15 K | 1737 °C | 3158,6 °F
θερμότητα σύντηξης
8 kJ/mol
Θερμότητα εξάτμισης
125 kJ/mol
Ειδική θερμοχωρητικότητα
- J/g·K
Αφθονία στον φλοιό της Γης
9,9×10-12%
Αφθονια στο συμπαν
χωρις στοιχεια
μοναδες CAS
7440-14-4
PubChem CID Number
6328144
ατομικές ιδιότητες
Ατομική ακτίνα
-
Ομοιοπολική ακτίνα
221 pm
Ηλεκτραρνητικότητα
0,9 (Κλίμακα Pauling)
Δυνατότητα ιοντισμού
5,2784 eV
Ατομικός Αριθμός
45,20 cm3/mol
Θερμική αγωγιμότητα
0,186 W/cm·K
Καταστάσεις οξείδωσης
2
εφαρμογές
Radium was formerly used in self-luminous paints for watches, nuclear panels, aircraft switches, clocks, and instrument dials.
Radium chloride was used in medicine to produce radon gas which in turn was used as a cancer treatment.
The isotope 223Ra is currently under investigation for use in medicine as a cancer treatment of bone metastasis.
Radium chloride was used in medicine to produce radon gas which in turn was used as a cancer treatment.
The isotope 223Ra is currently under investigation for use in medicine as a cancer treatment of bone metastasis.
Το ράδιο είναι ιδιαίτερα ραδιενεργό και καρκινογόνο
Ισότοπα
Σταθερά Ισότοπα
-ασταθη Ισότοπα
202Ra, 203Ra, 204Ra, 205Ra, 206Ra, 207Ra, 208Ra, 209Ra, 210Ra, 211Ra, 212Ra, 213Ra, 214Ra, 215Ra, 216Ra, 217Ra, 218Ra, 219Ra, 220Ra, 221Ra, 222Ra, 223Ra, 224Ra, 225Ra, 226Ra, 227Ra, 228Ra, 229Ra, 230Ra, 231Ra, 232Ra, 233Ra, 234Ra