Πρωτακτίνιο
91
Pa
Ομάδα
χωρις στοιχεια
Περίοδος
7
Τομέας
f
Πρωτονίων
ηλεκτρονικά
Νετρονίων
91
91
140
Γενικές Ιδιότητες
Ατομικός Αριθμός
91
Ατομικό βάρος
231,03588
αριθμός μάζας
231
Κατηγορία
Ακτινίδες
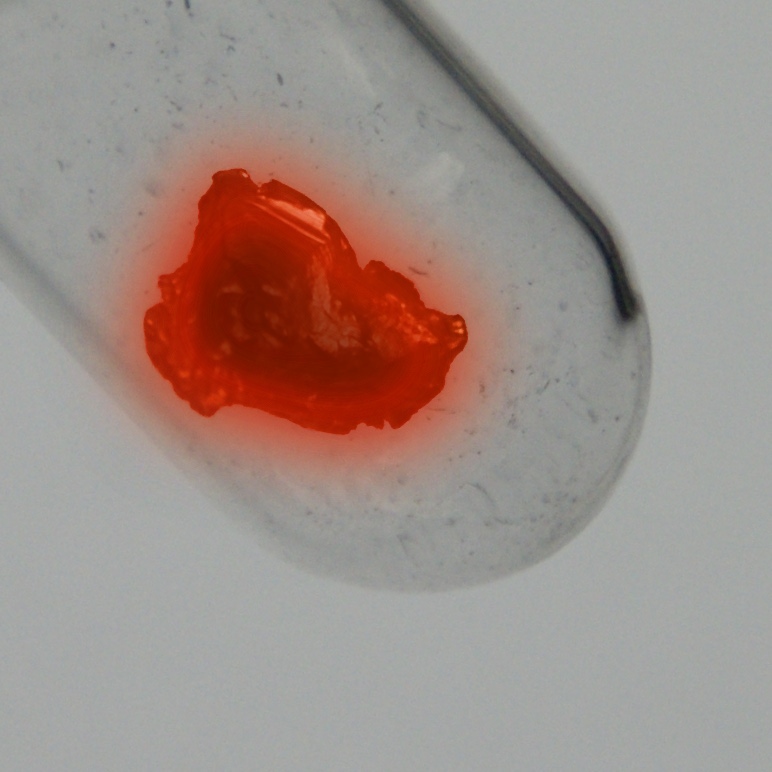
Χρώμα
ασημί
ραδιενεργο
Ναι
From the Greek protos meaning first
Κρυσταλλικό σύστημα
Κεντρικό Τετραγωνικό
Ιστορία
In 1900, William Crookes isolated protactinium as an intensely radioactive material from uranium
Protactinium was first identified in 1913 by Kasimir Fajans and Oswald Helmuth Göhring in Germany.
A more stable isotope of protactinium was discovered in 1917 by Otto Hahn and Lise Meitner at the Kaiser Wilhelm Institute in Berlin.
Protactinium was first identified in 1913 by Kasimir Fajans and Oswald Helmuth Göhring in Germany.
A more stable isotope of protactinium was discovered in 1917 by Otto Hahn and Lise Meitner at the Kaiser Wilhelm Institute in Berlin.
Ηλεκτρόνια ανά κέλυφος
2, 8, 18, 32, 20, 9, 2
Ηλεκτρονική διαμόρφωση
[Rn] 5f2 6d1 7s2
Protactinium is one of the rarest and most expensive naturally occurring elements
φυσικές ιδιότητες
φαση
Στερεά
Πυκνότητα
15,37 g/cm3
Σημείο τήξης
1841,15 K | 1568 °C | 2854,4 °F
Σημείο βρασμού
4300,15 K | 4027 °C | 7280,6 °F
θερμότητα σύντηξης
15 kJ/mol
Θερμότητα εξάτμισης
470 kJ/mol
Ειδική θερμοχωρητικότητα
- J/g·K
Αφθονία στον φλοιό της Γης
9,9×10-13%
Αφθονια στο συμπαν
χωρις στοιχεια
μοναδες CAS
7440-13-3
PubChem CID Number
χωρις στοιχεια
ατομικές ιδιότητες
Ατομική ακτίνα
163 pm
Ομοιοπολική ακτίνα
200 pm
Ηλεκτραρνητικότητα
1,5 (Κλίμακα Pauling)
Δυνατότητα ιοντισμού
5,89 eV
Ατομικός Αριθμός
15,0 cm3/mol
Θερμική αγωγιμότητα
0,47 W/cm·K
Καταστάσεις οξείδωσης
3, 4, 5
εφαρμογές
Owing to its scarcity, high radioactivity and high toxicity, there are currently no uses for protactinium outside of scientific research.
With the advent of highly sensitive mass spectrometers, an application of 231Pa as a tracer in geology and paleoceanography has become possible.
Protactinium-231 combined with the thorium-230 can be used to date marine sediments.
With the advent of highly sensitive mass spectrometers, an application of 231Pa as a tracer in geology and paleoceanography has become possible.
Protactinium-231 combined with the thorium-230 can be used to date marine sediments.
Το πρωτακτίνιο είναι τοξικό και ιδιαίτερα ραδιενεργό
Ισότοπα
Σταθερά Ισότοπα
-ασταθη Ισότοπα
212Pa, 213Pa, 214Pa, 215Pa, 216Pa, 217Pa, 218Pa, 219Pa, 220Pa, 221Pa, 222Pa, 223Pa, 224Pa, 225Pa, 226Pa, 227Pa, 228Pa, 229Pa, 230Pa, 231Pa, 232Pa, 233Pa, 234Pa, 235Pa, 236Pa, 237Pa, 238Pa, 239Pa, 240Pa