Μπερκέλιο
97
Bk
Ομάδα
χωρις στοιχεια
Περίοδος
7
Τομέας
f
Πρωτονίων
ηλεκτρονικά
Νετρονίων
97
97
150
Γενικές Ιδιότητες
Ατομικός Αριθμός
97
Ατομικό βάρος
[247]
αριθμός μάζας
247
Κατηγορία
Ακτινίδες
Χρώμα
χωρις στοιχεια
ραδιενεργο
Ναι
Named after Berkeley, California, the city of its discovery
Κρυσταλλικό σύστημα
απλο εξάγωνο
Ιστορία
Berkelium was discovered by Glenn T. Seaborg, Albert Ghiorso and Stanley G. Thompson in 1949 at the University of California, Berkeley.
It was produced by the bombardment of americium with alpha particles.
Berkelium was isolated in greater quantities for the first time by Burris Cunningham and Stanley Thompson in 1958.
It was produced by the bombardment of americium with alpha particles.
Berkelium was isolated in greater quantities for the first time by Burris Cunningham and Stanley Thompson in 1958.
Ηλεκτρόνια ανά κέλυφος
2, 8, 18, 32, 27, 8, 2
Ηλεκτρονική διαμόρφωση
[Rn] 5f9 7s2
Just over one gram of berkelium has been produced in the United States since 1967
φυσικές ιδιότητες
φαση
Στερεά
Πυκνότητα
14,78 g/cm3
Σημείο τήξης
1259,15 K | 986 °C | 1806,8 °F
Σημείο βρασμού
3173,15 K | 2900 °C | 5252 °F
θερμότητα σύντηξης
χωρις στοιχεια kJ/mol
Θερμότητα εξάτμισης
χωρις στοιχεια kJ/mol
Ειδική θερμοχωρητικότητα
- J/g·K
Αφθονία στον φλοιό της Γης
χωρις στοιχεια
Αφθονια στο συμπαν
χωρις στοιχεια
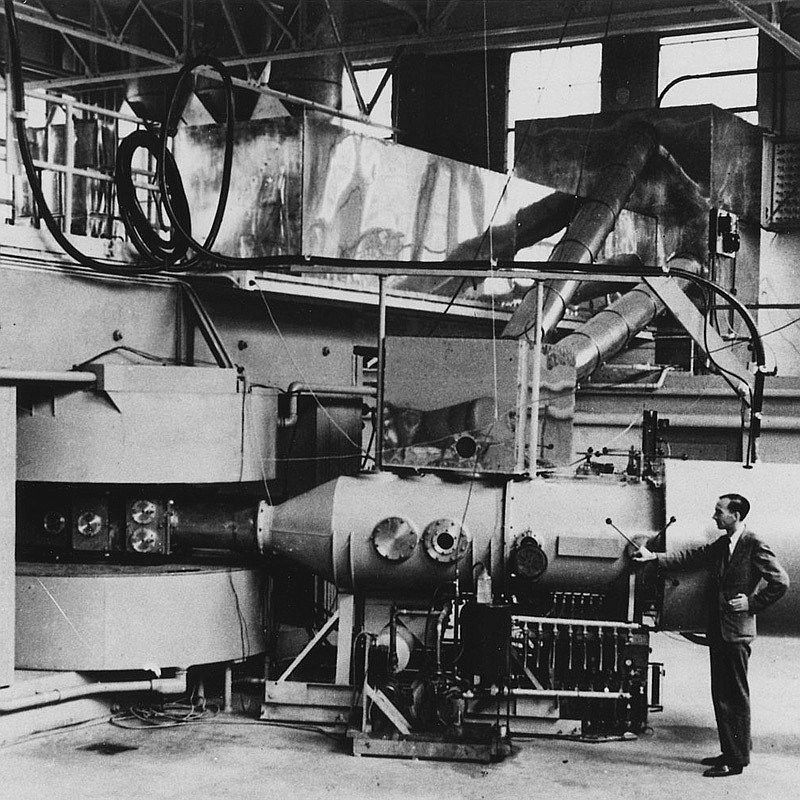
πιστωεις εικονας: Wikimedia Commons (Department of Energy - Office of Public Affairs)
The 60-inch cyclotron at the Lawrence Radiation Laboratory, University of California, Berkeley
μοναδες CAS
7440-40-6
PubChem CID Number
23971
ατομικές ιδιότητες
Ατομική ακτίνα
170 pm
Ομοιοπολική ακτίνα
-
Ηλεκτραρνητικότητα
1,3 (Κλίμακα Pauling)
Δυνατότητα ιοντισμού
6,1979 eV
Ατομικός Αριθμός
16,7 cm3/mol
Θερμική αγωγιμότητα
0,1 W/cm·K
Καταστάσεις οξείδωσης
3, 4
εφαρμογές
Berkelium is mainly used for scientific research purposes.
Berkelium-249 is a common target nuclide to prepare still heavier transuranic elements and transactinides, such as lawrencium, rutherfordium and bohrium.
It is also useful as a source of the isotope californium-249.
Berkelium-249 is a common target nuclide to prepare still heavier transuranic elements and transactinides, such as lawrencium, rutherfordium and bohrium.
It is also useful as a source of the isotope californium-249.
Το βερκέλιο είναι επιβλαβές λόγω της ραδιενέργειάς του
Ισότοπα
Σταθερά Ισότοπα
-ασταθη Ισότοπα
233Bk, 235Bk, 236Bk, 237Bk, 238Bk, 239Bk, 240Bk, 241Bk, 242Bk, 243Bk, 244Bk, 245Bk, 246Bk, 247Bk, 248Bk, 249Bk, 250Bk, 251Bk, 252Bk, 253Bk, 254Bk